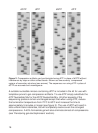
7
uses dideoxynucleoside triphosphates, generating uniform band intensities in
sequencing experiments (with dGTP). These properties make the enzyme ideal
for generating high-quality DNA sequences using cycle-sequencing methods. It
is stable at 90°C for at least 1 hour and retains 50% of its activity when
incubated at 95°C for 60 minutes. The Thermo Sequenase polymerase in this
kit combines the advantages of both Sequenase DNA polymerase and
Taq
DNA polymerase. It produces bands (with Mg
2+
) that are nearly as uniform as
those produced with Sequenase DNA polymerase with Mn
2+
(10), yet is
thermostable like
Taq
DNA polymerase.
Cycle sequencing is the name given to the process of using repeated cycles of
thermal denaturation, primer annealing, and polymerization to produce greater
amounts of product in a DNA sequencing reaction. This amplification process
employs a single primer so the amount of product DNA increases linearly with
the number of cycles. (This distinguishes it from PCR* which uses 2 primers so
that the amount of product can increase exponentially with the number of
cycles.)
The earliest examples of cycle sequencing used
32
P-labeled primers and a non-
thermostable polymerase which was added after each denaturation cycle
(11,12). Later improvements included the use of thermostable
Taq
polymerase
(13,14) and the use of alpha-labeled dNTPs in place of the labeled primer
using mixtures of nucleotides similar to those used originally by Sanger (15,16).
The labeled-primer methods make efficient use of
32
P giving a sequence with
as little as 4µCi of [γ-
32
P]ATP (14). The methods using internally-labeled
products were less efficient, requiring either 10µCi of [α-
33
P]dATP or 20µCi of
[α-
35
S]dATP for a sequence. This is a consequence of the relatively low specific
radioactivity and the small number of labeled bases in short product molecules.
This kit makes very efficient use of [α-
33
P]ddNTP, requiring less than 1µCi of
33
P per sequence. Cycle sequencing is necessary with this kit when using less
than 0.2-0.5pmol of template DNA. Non-cycle (or very few cycle) protocols may
be used with more than ~0.5pmol of template.
*See license information on back cover.