21
than 1pmol of template DNA for each sequence (0.25pmol per reaction).
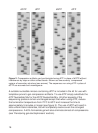
See figure 1.
3. G-C rich template producing strong secondary structure. Try less DNA,
longer extension times, more cycles, more enzyme, 5% DMSO, or a 96°C
denaturation temperature.
Film blank or very faint
1. If using single-sided film, the emulsion side must be placed facing the dried
gel.
2. DNA preparation may be bad. Try the control DNA supplied in the kit.
3. Labeled dideoxynucleotide too old. Try longer exposure.
4. Some component missing.
5. Enzyme lost activity.
6. Insufficient template DNA or insufficient number of cycles. Try more DNA,
more cycles or longer film exposure.
7. Incorrect temperatures for primers used. Try a lower temperature for cycling
(
e.g.
50°-95°C), especially when using dITP.
8. Incorrect termination time or temperature for dITP. Termination should be 5-
10 minutes at 55-60°C.
8. Too little primer used. The recommended amount of primer is 0.5-2.5pmol.
9. Primer bad. Some primers form dimers, hairpins etc., interfering with
annealing with the template. Try a different primer.
10.Wrong amounts of dNTP or [a-
33
P]ddNTP used. Check volumes added.
11.Large excess of primer and DNA used. Check quantities added to reaction.
Bands faint near the primer
1. Too much dNTP or too little [α-
33
P]ddNTP used. Check volumes added.
Bands smeared
1. Contaminated DNA preparation. Try control DNA. Thermo Sequenase DNA
polymerase is sensitive to salt concentration, especially above 75mM.
2. Gel may be bad. Gels should be cast with fresh acrylamide solutions and
should polymerize rapidly, within 15 minutes of pouring. Try running a
second gel with the same samples.
3. Gel run too cold. Sequencing gels should be run at 40-55°C.
4. Gel dried too hot or not flat enough to be evenly exposed to film.
5. Samples not denatured. Make sure samples are always heated to 70°C for
at least 2 minutes (longer in an air-filled heat block) immediately prior to
loading on gel. When re-loading a sample (
e.g.
for a second gel or a double-
loaded gel) the heating step should be repeated.
Bands appear across all 4 lanes
1. Gel compression artifacts. Sometimes a band in all 4 lanes indicates a
severe gel compression caused by secondary structures not completely
denatured during electrophoresis. If the gel has a region where the bands
are very closely spaced, followed by a region where the bands are widely