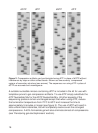
9
PROTOCOL
1. Termination mixes—Prepare the termination mixes on ice. Mix 2µl of
Nucleotide Master Mix (either dGTP or dITP—see note below) and 0.5µl of
[α-
33
P]ddNTP (G, A, T, or C—one of each per sequence) to produce a
termination mix for each ddNTP. Label, fill and cap four tubes (‘G’, ‘A’, ‘T’,
‘C’) with 2.5µl of each termination mix. It is more accurate and convenient
to prepare batches of termination mixes sufficient for all sequences to
be performed, then dispense 2.5µl from this batch to each vial for the
termination reactions. It is recommended that these batches of termination
mixes be made up routinely.
To prepare termination mixes for (n) reactions, mix:
GATC
Nucleotide Master Mix (2 x n)µl (2 x n)µl (2 x n)µl (2 x n)µl
[α-
33
P]ddNTP (0.5 x n)µl (0.5 x n)µl (0.5 x n)µl (0.5 x n)µl
––––––––– ––––––––– ––––––––– –––––––––
Total (2.5 x n)µl (2.5 x n)µl (2.5 x n)µl (2.5 x n)µl
Note: The termination tubes can be left uncapped until all reagents have
been added if the tubes are kept on ice and the reaction mixture is added
within a few minutes. For determination of new sequences, or of sequences
with high G-C content, the dITP Nucleotide Master Mix is recommended. This
will eliminate all compression artifacts but will result in somewhat uneven
band intensities, especially in the ‘G’ lane. When perfectly uniform band
intensities are desired, such as when examining sequences from potentially
heterozygous individuals, the dGTP Nucleotide Master Mix should be used.
2. Reaction mixture:
For multiple (n) reactions with different primers and/or templates, prepare a
n+1 batch of reaction buffer, water, polymerase and aliquot; then add the
unique primer and/or template in the appropriate concentration and volume to
the aliquots.
Reaction Buffer 2µl
DNA _µl*(50-500ng or 25-250fmol)
Primer _µl*(0.5-2.5pmol)
H
2
O_µl (To adjust total volume to 20µl)
Thermo Sequenase polymerase (4U/µl) 2µl (8 units polymerase—add LAST)
–––
Total 20µl
*For the control reaction, use 10µl of control DNA and 1µl of control primer.