119
MODEL 3081 pH/ORP SECTION 13.0
pH MEASUREMENTS
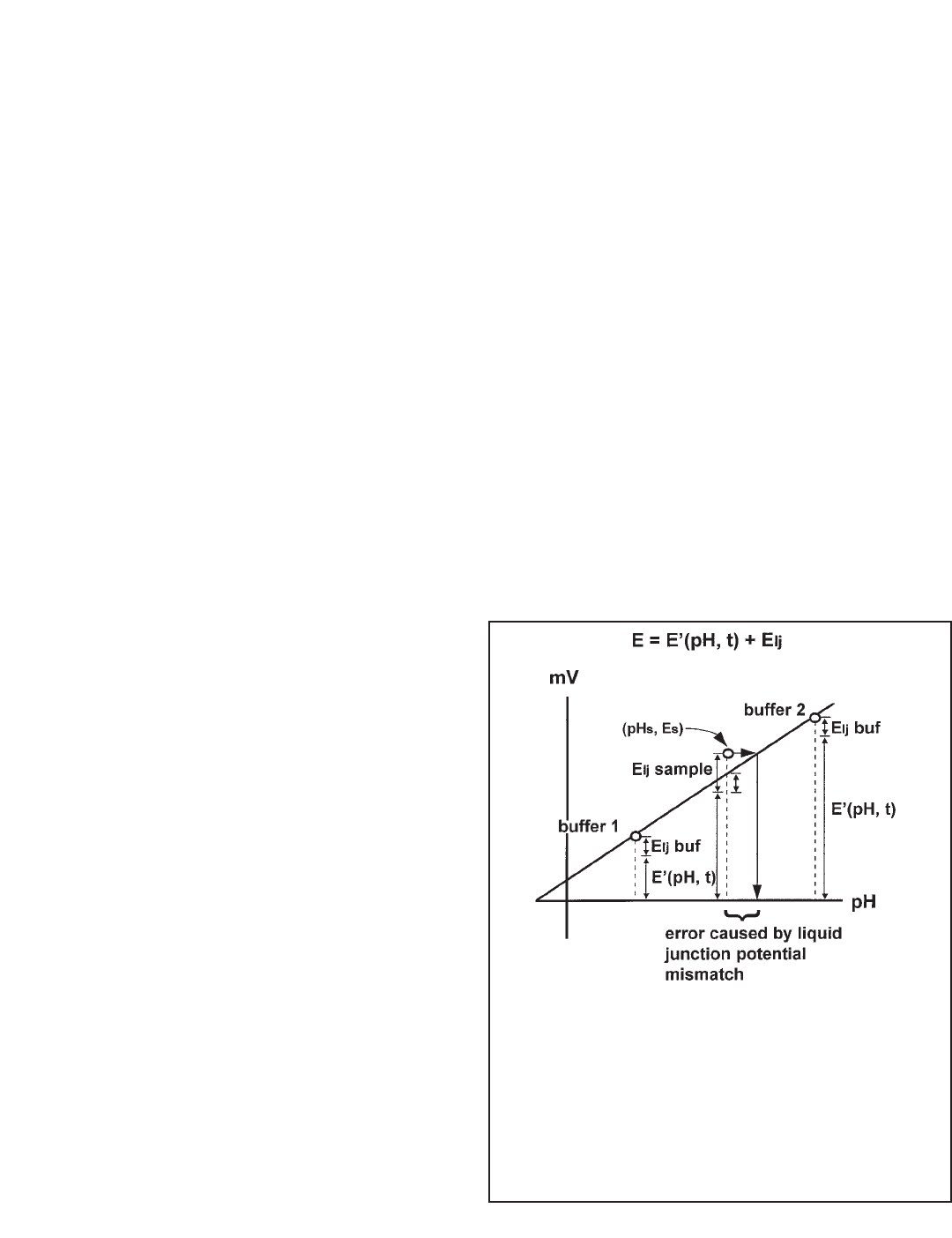
junction potentials in the buffers are assumed to be equal
and are exaggerated for clarity.
If the liquid junction potential in the sample differs from the
buffers, a measurement error results. Figure 13-8 illus-
trates how the error comes about. Assume the true pH of
the sample is pH
s
and the cell voltage is E
s
. The point (pH
s
,
E
s
) is shown on the graph. If the liquid junction potential in
the sample were equal to the value in the buffers, the point
would lie on the line. However, the liquid junction potential
in the sample is greater, so the point E
s
lies above the cal-
ibration line. Therefore, when the cell voltage is converted
to pH, the result is greater than the true pH by the amount
shown.
A typical mismatch between liquid junction potentials in
buffer and sample is 2-3 mV, which is equivalent to an error
of about ±0.02 pH units. The mismatch produces a funda-
mental error in pH determinations using a cell with liquid
junction.
13.10 SENSOR DIAGNOSTICS
Sensor diagnostics alert the user to problems with the sen-
sor or to actual sensor failures. The two sensor diagnostics
are reference impedance and glass impedance.
The major contributor to reference impedance is the resist-
ance across the liquid junction plug. In a properly function-
ing electrode, the resistance of the liquid junction should be
no more than several hundred kilohms. If the junction is
plugged or if the filling solution or gel is depleted, the resist-
ance increases. A high reference impedance may also
mean the sensor is not immersed in the process stream.
Glass impedance refers to the impedance of the pH-sensi-
tive glass membrane. The impedance of the glass mem-
brane is a strong function of temperature. As temperature
increases, the impedance decreases. For a change in
glass impedance to have any meaning, the impedance
measurement must be corrected to a reference tempera-
ture. The impedance of a typical glass electrode at 25°C is
several hundred megohms. A sharp decrease in the tem-
perature-corrected impedance implies that the glass is
cracked. A cracked glass electrode produces erroneous pH
readings. The electrode should be replaced immediately. A
high temperature-corrected glass impedance implies the
sensor is nearing the end of its life and should be replaced
as soon as possible.
13.11 SHIELDS, INSULATION, AND
PREAMPLIFIERS
pH measurement systems, cell and meter, have high
impedance. The high impedance circuit imposes important
restrictions on how pH measurement systems are
designed.
The lead wire from the glass electrode connects two high
resistances: about 100 MΩ at the electrode and about
1,000,000 MΩ at the meter. Therefore, electrostatic
charges, which accumulate on the wire from environmental
influences, cannot readily drain away. Buildup of charge
results in degraded, noisy readings. Shielding the wire with
metal braid connected to ground at the instrument is one
way to improve the signal. It is also helpful to keep the sen-
sor cable as far away as possible from AC power cables.
The high input impedance of the pH meter requires that the
lead insulation and the insulation between the meter inputs
be of high quality. To provide further protection from envi-
ronmental interference, the entire sensor cable can be
enclosed in conduit.
To avoid the need for expensive cable and cable installa-
tions, a preamplifier built into the sensor or installed in a
junction box near the sensor can be used. The preamplifi-
er converts the high impedance signal into a low imped-
ance signal that can be sent as far as 200 feet without spe-
cial cable.
FIGURE 13-8. Liquid Junction Potential Mismatch.
The dashed vertical lines are the measured cell voltages for
the buffers and the sample. The contribution from each
term in equation 4 is shown. The buffers are are assumed
to have identical liquid junction potentials. Because most
buffers are equitransferant, i.e., the mobilities of the ions
making up the buffer are nearly equal, assuming equal liq-
uid junction potentials is reasonable. In the figure, the liquid
junction potential of the sample is greater than the buffers.
The difference gives rise to an error in the measured pH.