122
Liquid junction potentials exist whenever dissimilar elec-
trolyte solutions come into contact. The magnitude of the
potential depends on the difference between the mobility of
the ions. Although liquid junction potentials cannot be elim-
inated, they can be made small and relatively constant. A
small liquid junction potential exists when the ions present
in greatest concentration have equal (or almost equal)
mobilities. The customary way of reducing junction poten-
tials is to fill the reference electrode with concentrated
potassium chloride solution. The high concentration
ensures that potassium chloride is the major contributor to
the junction potential, and the nearly equal mobilities of
potassium and chloride ions makes the potential small.
14.5 RELATING CELL VOLTAGE TO ORP
The measured cell voltage, E(T)—the notation emphasizes
the temperature dependence—is the algebraic sum of the
measuring (platinum) electrode potential, the reference
electrode potential, and the liquid junction potential.
Because the potential of the reference electrode is inde-
pendent of ORP and the liquid junction potential is small,
the measured cell voltage is controlled by the ORP of the
sample. Stated another way, the cell voltage is the ORP of
the sample relative to the reference electrode.
14.6 ORP, CONCENTRATION, AND pH
ORP depends on the relative concentration of oxidized and
reduced substances in the sample and on the pH of the
sample. An understanding of how concentration and pH
influence ORP is necessary for the correct interpretation of
ORP readings.
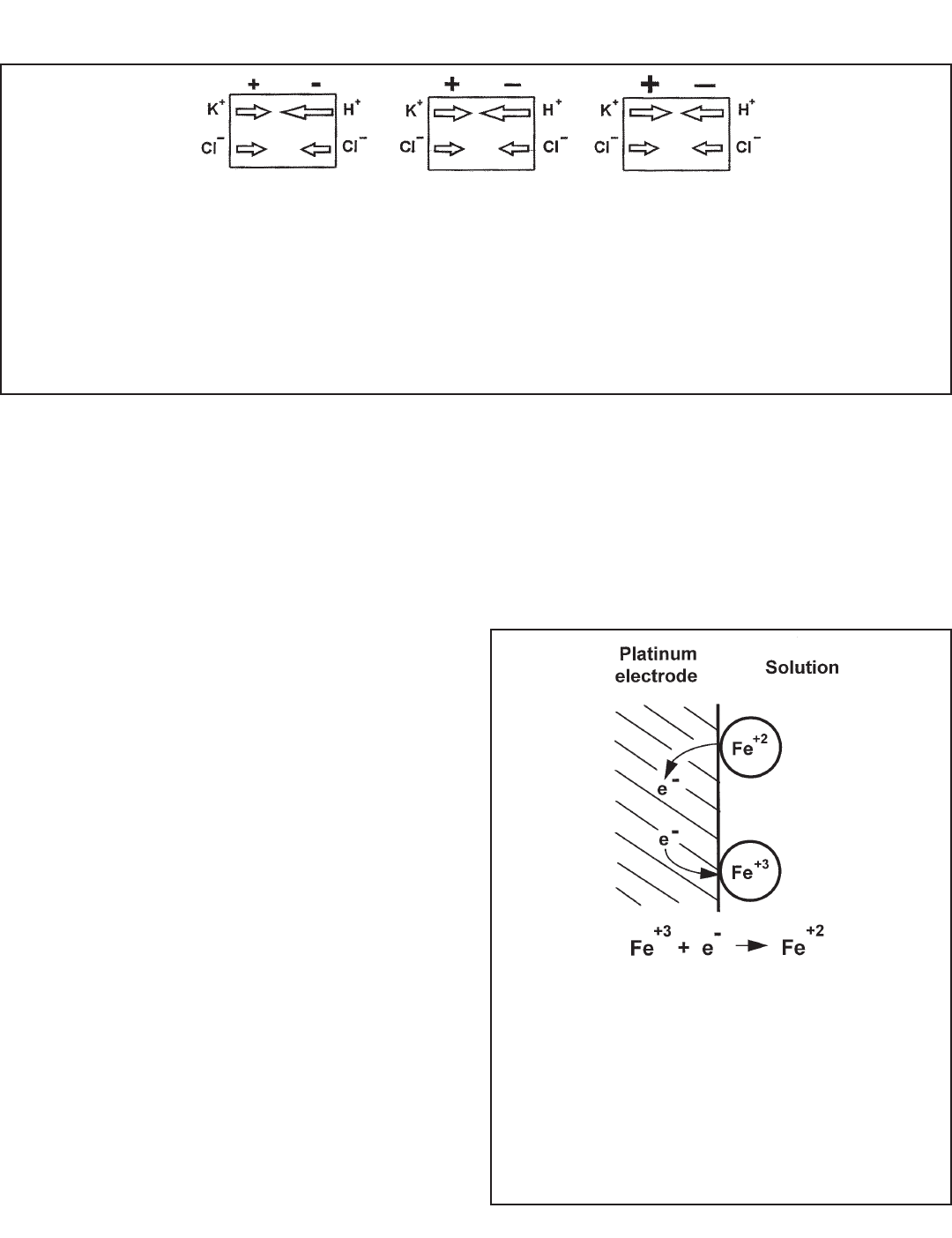
Figure 14-5 shows a platinum ORP electrode in contact
with a solution of iron (II) and iron (III). As discussed earli-
er, iron (II) and iron (III) are a redox couple. They are relat-
ed by the following half reaction:
Fe
+3
+ e
-
= Fe
+2
(1)
If a redox couple is present, a stable electrical potential
eventually develops at the interface between the platinum
electrode and the sample. The magnitude of the potential
MODEL 3081 pH/ORP SECTION 14.0
ORP MEASUREMENTS
FIGURE 14-4. The Origin of Liquid Junction Potentials.
The figure shows a thin section through a pore in the junction plug. The junction separates a solution of potassium chloride
on the left from a solution of hydrochloric acid on the right. The solutions have equal molar concentration. Driven by con-
centration differences, hydrogen ions and potassium ions diffuse in the directions shown. The length of each arrow indicates
relative rates. Because hydrogen ions move faster than potassium ions, positive charge builds up on the left side of the sec-
tion and negative charge builds up on the right side. The ever-increasing positive charge repels hydrogen and potassium
ions. The ever-increasing negative charge attracts the ions. Therefore, the migration rate of hydrogen decreases, and the
migration rate of potassium increases. Eventually the rates become equal. Because the chloride concentrations are the
same, chloride does not influence the charge separation or the liquid junction potential.
FIGURE 14-5. Electrode Potential.
The drawing shows an iron (II) and iron (III) ion at the sur-
face of a platinum electrode. Iron (III) can take an electron
from the platinum and be reduced, and iron (II) can place
an electron on the metal and be oxidized. The electrode
potential is the tendency of the half reaction shown in the
figure to occur spontaneously. Because the voltmeter
used to measure ORP draws almost no current, there is
no change in the concentration of iron (II) and iron (III) at
the electrode.


















