123
is described by the following equation, called the
Nernst equation:
E = E° - (2)
In the Nernst equation, E is the electrode potential and
E° is the standard electrode potential, both in millivolts,
t is temperature in °C, n is the number of electrons
transferred (n = 1 in the present case), and [Fe
+2
] and
[Fe
+3
] are the concentrations of iron (II) and iron (III)
respectively. There are several ways of defining the
standard electrode potential, E°. No matter which defi-
nition is used, the standard electrode potential is sim-
ply the electrode potential when the concentrations of
iron (II) and iron (III) have defined standard values.
Equation 2 shows that the electrode potential is con-
trolled by the logarithm of the ratio of the concentration
of iron (II) to iron (III). Therefore, at 25°C if the ratio
changes by a factor of ten, the electrode potential
changes by
- log 10 = - 59.2 mV
As the expression above shows, the voltage change is
also directly proportional to temperature and inversely
proportional to the number of electrons transferred.
14.7 INTERPRETING ORP MEASUREMENTS
Interpreting ORP and changes in ORP requires great
caution. There are several concepts to keep in mind
concerning industrial ORP measurements.
• ORP is best used to track changes in concentration or
to detect the presence or absence of certain chemicals.
For example, in the treatment of wastes from metal fin-
ishing plants, chromium (VI) is converted to chromium
(III) by treatment with sulfur dioxide. Because chromium
(VI) and chromium (III) are a redox couple, ORP can be
used to monitor the reaction. As sulfur dioxide converts
chromium (VI) to chromium (III), the concentration ratio
changes and the ORP drops. Once all the chromium
(VI) has been converted to chromium (III) and a slight
excess of sulfur dioxide is present, the chromium cou-
ple no longer determines ORP. Instead, ORP is con-
trolled by the sulfur dioxide-sulfate couple. When sulfur
dioxide reacts with chromium (VI), it is converted to sul-
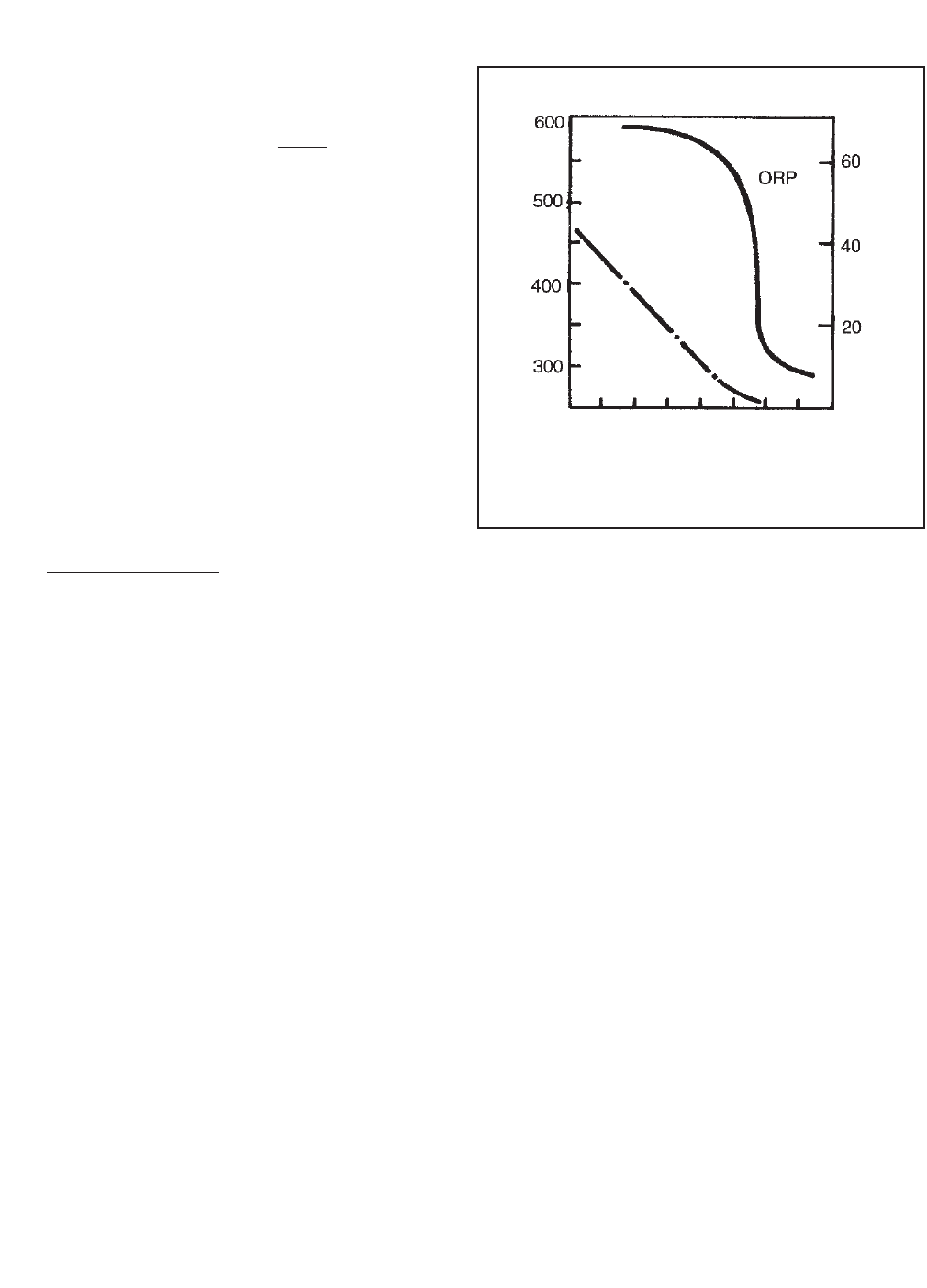
fate. Figure 14-6 shows how ORP and the concentra-
tion of chromium (VI) change as sulfur dioxide is added.
Because the change in ORP at the endpoint is large,
monitoring ORP is an efficient way of tracking the
process.
• ORP measures activity, not concentration. Activity
accounts for the way in which other ions in solution
influence the behavior of the redox couple being meas-
ured. To be strictly correct, ORP is controlled by the the
ratio of activities, not concentrations. The dependence
of ORP on activity has an important consequence.
Suppose a salt, like sodium sulfate, is added to a solu-
tion containing a redox couple, for example iron (II) and
iron (III). The sodium sulfate does not change the con-
centration of either ion. But, the ORP of the solution
does change because the salt alters the ratio of the
activity of the ions.
• pH can have a profound influence on ORP. Referring
to the earlier example where ORP was used to monitor
the conversion of chromium (VI) to chromium (III). The
reaction is generally carried out at about pH 2.
Because the concentration ratio in the Nernst equation
also includes hydrogen ions, the ORP of a mixture of
chromium (VI) and chromium (III) is a function of pH.
To appreciate the extent to which pH influences ORP,
consider the conversion of chromium (VI) to chromium
(III). In acidic solution the half reaction is:
Cr
2
O
7
-2
+ 14 H
+
+ 6 e
-
= 2 Cr
+3
+ 7 H
2
O (3)
• Chromium (VI) exists as dichromate, Cr
2
O
7
-2
, in
acidic solution.
MODEL 3081 pH/ORP SECTION 14.0
ORP MEASUREMENTS
FIGURE 14-6. ORP Measurement Interpretation
0.1987 (t + 273.15)
log
[Fe
+2
]
n [Fe
+3
]
0.1987 (25 + 273.15)
1
Sulfur dioxide added
Cr (VI)
ORP, mV
Chromium (VI), ppm


















