Principles of Operation Teledyne API - Model T200H/T200M Operation Manual
268
νhNONO +
2
*
2
(Equation 9-2)
All things being constant, the relationship between the amount of NO present in the
reaction cell and the amount of light emitted from the reaction is very linear. More NO
produces more light, which can be measured with a light-sensitive sensor in the near-
infrared spectrum (Figure 8-1). In order to maximize the yield of reaction (1), the
T200H/M supplies the reaction cell with a large, con
stant excess of ozone (about 3000-
5000 ppm) from the internal ozone generator.
Model 200E Instrument Response
0 a.u.
20 a.u.
40 a.u.
60 a.u.
80 a.u.
100 a.u.
120 a.u.
140 a.u.
0.5µm 0.7µm 0.9µm 1.1µm 1.3µm 1.5µm 1.7µm 1.9µm
Wavelength
Intensit
y
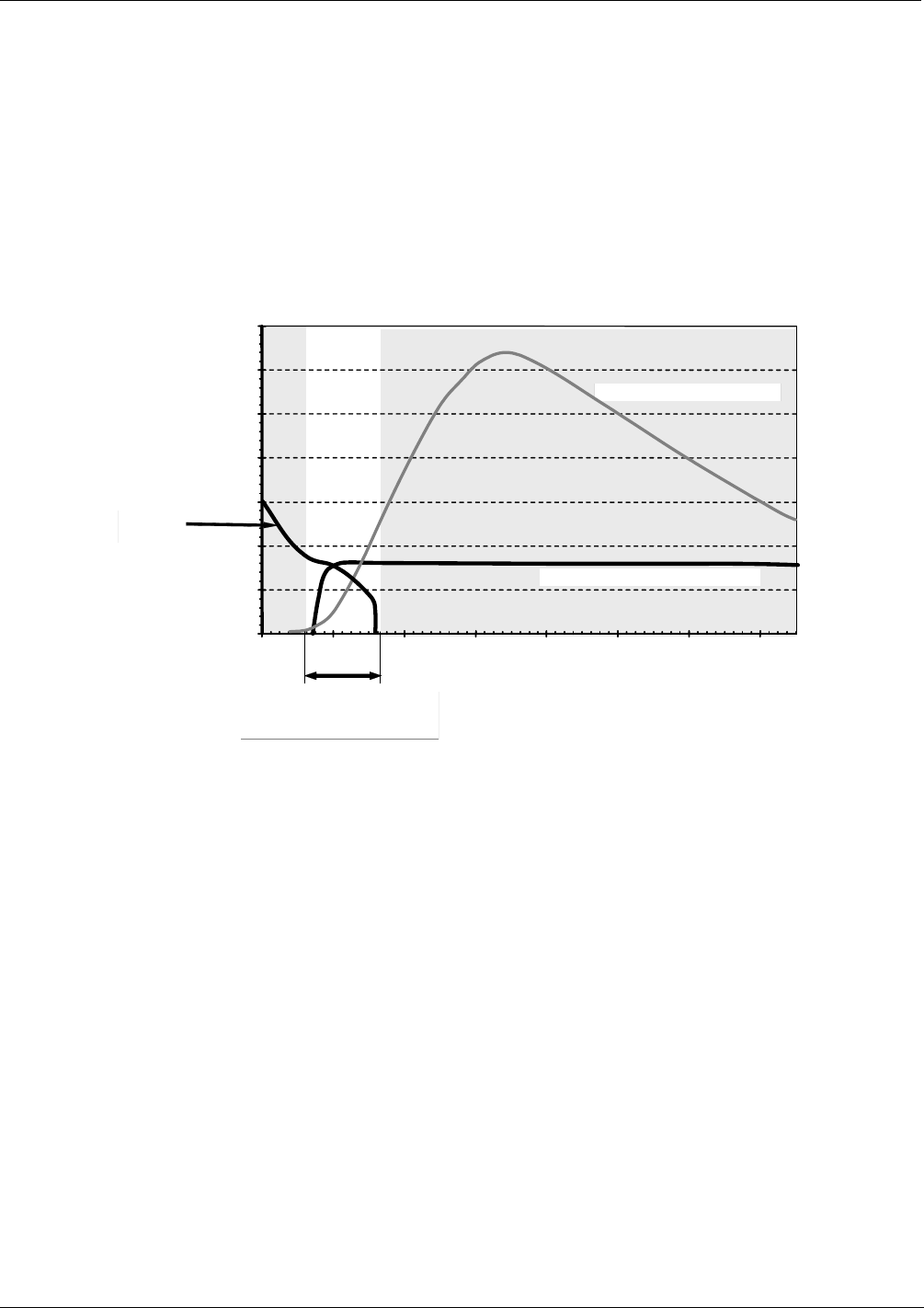
Optical Hi-Pass Filter Performance
NO + O
3
Emission Spectrum
PMT
Response
M200EH/EM
Sensitivity Window
Figure 8-1: T200H/M Sensitivity Spectrum
However, only about 20% of the NO
2
that is formed through reaction 10-1 is in the
excited state. In addition, the excited NO
2
can collide with another collision partner M
in the reaction cell (mostly other molecules but also cell walls) and transfer its excess
energy to its collision partner without emitting any light at all (Equation 9-3). In fact, by
far the largest portion of the NO
2
* returns to the ground state this way, leaving only a
few percent yield of usable chemiluminescence.
MNOMNO ++
2
*
2
(Equation 9-3)
In order to enhance the light yield of the reaction, the reaction cell is maintained at
reduced pressure. The probability of a collision between the NO
2
* molecule and a
collision partner M increases proportionally with the reaction cell pressure. This non-
radiating collision with the NO
2
* molecules is usually referred to as quenching, an
unwanted process further described in Section 8.2.4.2.
07270B DCN6512


















