Teledyne API - Model T200H/T200M Operation Manual Principles of Operation
273
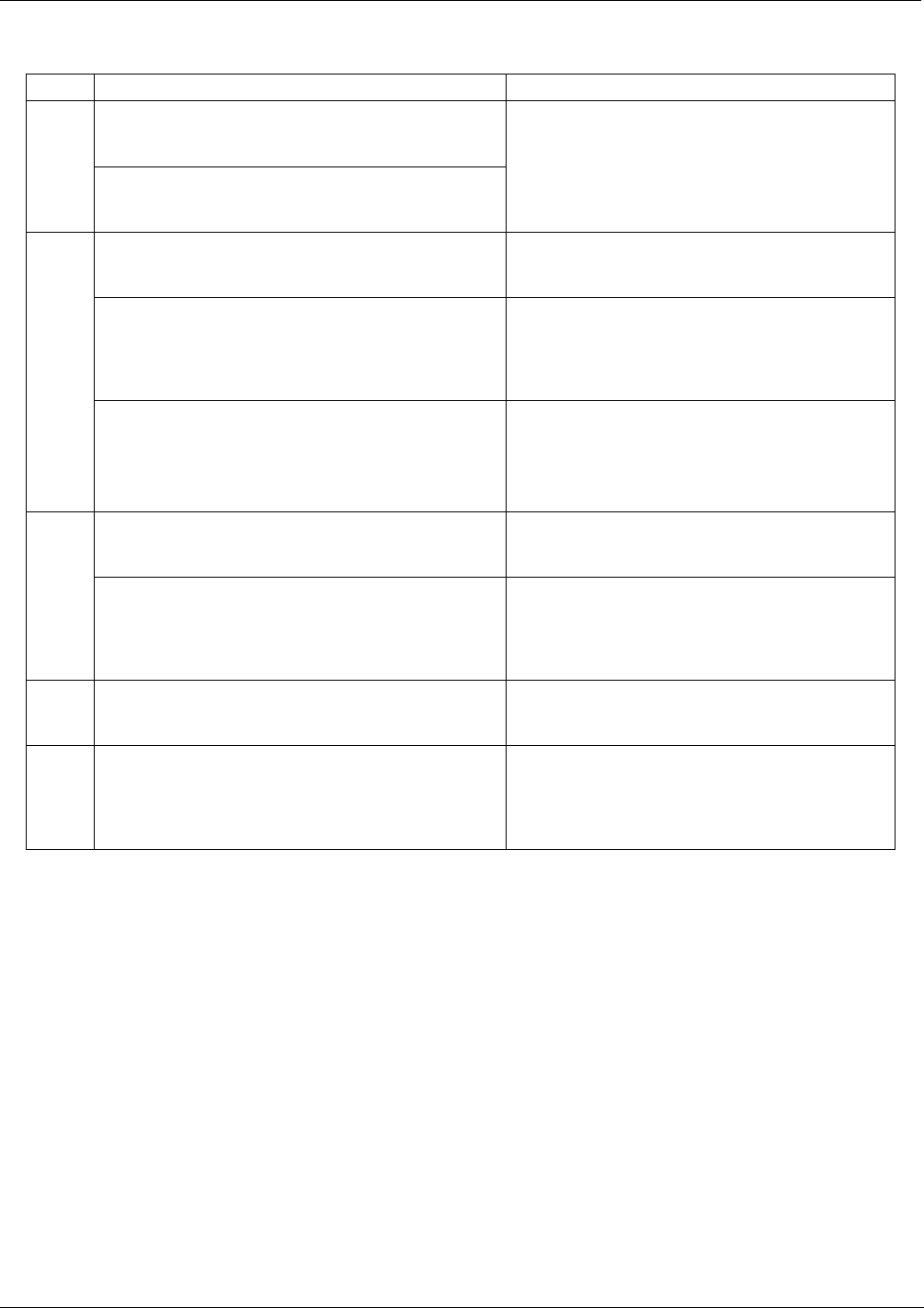
Table 8-1: List of Interferents
GAS INTERFERENCE TYPE REJECTION METHOD
Dilution: Viscosity of CO
2
molecules causes them to
collect in aperture of Critical Flow Orifice altering flow
rate of NO.
CO
2
3
rd
Body Quenching: CO
2
molecules collide with
NO
2
* molecules absorbing excess energy kinetically
and preventing emission of photons.
If high concentrations of CO
2
are suspected,
special calibration methods must be performed to
account for the affects of the CO
2
.
Contact Teledyne API Technical Support depart-
ment for details.
Some SO
X
variants can also initiate a
chemiluminescence reaction upon exposure to O
3
producing excess light.
Wavelengths of light produced by
chemiluminescence of SO
X
are screened out by
the Optical Filter.
Chemically reacts with NH
3
, O
2
and H
2
O in O
3
generator to create (NH
3
)
2
SO
4
(ammonium sulfate)
and NH
3
NO
2
(ammonium nitrate) which form opaque
white deposits on optical filter window. Also forms
highly corrosive HNO
3
(Nitric Acid)
Most of the ammonium sulfate and ammonium
nitrate produced is removed from the sample gas
by an air purifier located between the O
3
Generator and the reaction cell.
SO
X
3
rd
Body quenching: SO
X
molecules collide with NO
2
*
molecules absorbing excess energy kinetically and
preventing emission of photons.
If high concentrations of SO
X
are suspected,
special calibration methods must be performed to
account for the affects of the SO
2
.
Contact Teledyne API Technical Support depart-
ment for details.
3
rd
Body quenching: H
2
O molecules collide with NO
2
*
molecules absorbing excess energy kinetically and
preventing emission of photons.
Analyzer’s operating in high humidity areas must
have some method of drying applied to the
sample gas supply (Section 5.10 for more details).
H
2
0
Chemically reacts with NH
3
and SO
X
in O
3
generator
to create (NH
3
)
2
SO
4
(ammonium sulfate) and
NH
3
NO
2
(ammonium nitrate) which form opaque
white deposits on optical filter Window. Also forms
highly corrosive HNO
3
(nitric acid)
Removed from the O
3
gas stream by the Perma
Pure
®
Dryer (Section 8.3.7 for more details).
NH
3
Direct Interference: NH
3
is converted to H
2
O and NO
by the NO
2
converter. Excess NO reacts with O
3
in
reaction cell creating excess chemiluminescence.
If a high concentration of NH
3
is suspected, steps
must be taken to remove the NH
3
from the sample
gas prior to its entry into the NO
2
converter.
Chemically reacts with H
2
O, O
2
and SO
X
in O
3
generator to create (NH
3
)
2
SO
4
(ammonium sulfate)
and NH
3
NO
2
(ammonium nitrate) which form opaque
white deposits on optical filter window. Also forms
highly corrosive HNO
3
(nitric acid).
The Perma Pure
®
dryer built into the T200H/M is
sufficient for removing typical ambient
concentration levels of NH
3
.
In cases with excessively high CO
2
concentrations (larger than 0.5%), the effect can be
calibrated out by using calibration gases with a CO
2
content equal to the measured air.
Only very high and highly variable CO
2
concentrations will then be cause of measurable
interference. For those applications, we recommend to use other analyzer models.
Please consult sales or our website.
8.2.4.3. Light Leaks
The T200H/M sensitivity curve includes a small portion of the visible light spectrum
(Figure 10-1), hence, it is important to make sure than the reaction cell is completely
sealed with respect to light. To ensure this, all pneumatic tubing leading into the
reaction cell is either opaque (vacuum exit tubing) in order to prevent light from entering
the cell or light penetration is prevented by stainless steel filters and orifices (gas
entries).
07270B DCN6512


















